Important Safety Information: The Pulmonx Zephyr® Endobronchial Valves are implantable bronchial valves indicated for the bronchoscopic treatment of adult patients with hyperinflation associated with severe emphysema in regions of the lung that have little to no collateral ventilation. Complications can include but are not limited to pneumothorax (tear in the lung), worsening of COPD symptoms, hemoptysis, pneumonia, and, in rare cases, death. The Zephyr Valve is contraindicated in patients who have not quit smoking. Please talk with your physician about other contraindications, warnings, precautions, and adverse events. Only a trained physician can decide whether a particular patient is an appropriate candidate for treatment with the Zephyr Valve.
Caution: Federal law restricts this device to sale by or on the order of a physician.
© 2021-Present Pulmonx Corporation or its affiliates. All rights reserved. All trademarks herein are the property of Pulmonx Corporation and its affiliates. US-EN-405-v5
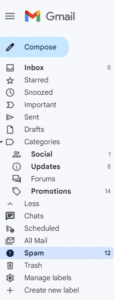
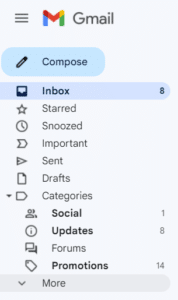 If you do not see an email from “Val at Zephyr Valve” in your inbox within 15 minutes of signing up, go to your “Spam” folder within Gmail. (You may have to click "More" to see the Spam folder.) Select the email from "Val at Zephyr Valve."
There will be a gray box at the top that says “Why is this message in spam?” Click the “Report Not Spam” button. This will move the email to your inbox.
If you do not see an email from “Val at Zephyr Valve” in your inbox within 15 minutes of signing up, go to your “Spam” folder within Gmail. (You may have to click "More" to see the Spam folder.) Select the email from "Val at Zephyr Valve."
There will be a gray box at the top that says “Why is this message in spam?” Click the “Report Not Spam” button. This will move the email to your inbox.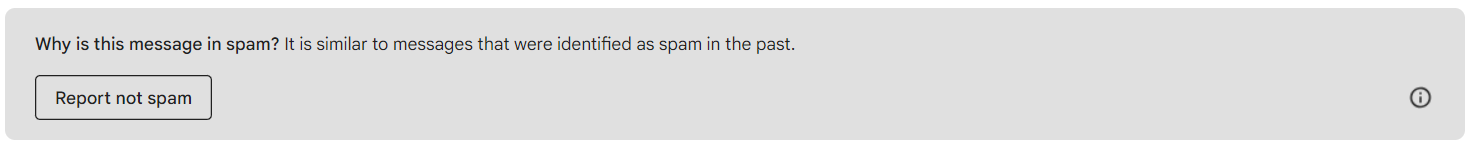 Go back to your inbox and open the email. Click on the icon next to “Val at Zephyr Valve”, you’ll see a person icon with a “+”. Click that, and you can add Val to your Contacts list. If you don’t receive a second email from us in a few days, please check your Spam folder again.
Go back to your inbox and open the email. Click on the icon next to “Val at Zephyr Valve”, you’ll see a person icon with a “+”. Click that, and you can add Val to your Contacts list. If you don’t receive a second email from us in a few days, please check your Spam folder again.
